Alternative Reactivity of Leucine 5-Hydroxylase Using an Olefin-Containing Substrate to Construct a Substituted Piperidine Ring
Abstract
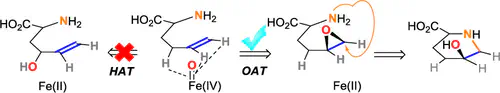
Applying enzymatic reactions to produce useful molecules is a central focus of chemical biology. Iron and 2-oxoglutarate (Fe/2OG) enzymes are found in all kingdoms of life and catalyze a broad array of oxidative transformations. Herein, we demonstrate that the activity of an Fe/2OG enzyme can be redirected when changing the targeted carbon hybridization from sp3 to sp2. During leucine 5-hydroxylase catalysis, installation of an olefin group onto the substrate redirects the Fe(IV)–oxo species reactivity from hydroxylation to asymmetric epoxidation. The resulting epoxide subsequently undergoes intramolecular cyclization to form the substituted piperidine, 2S,5S-hydroxypipecolic acid.
Type
Publication
Biochemistry
Supplementary Information is here, including data.