Deciphering pyrrolidine and olefin formation mechanism in kainic acid biosynthesis
Abstract
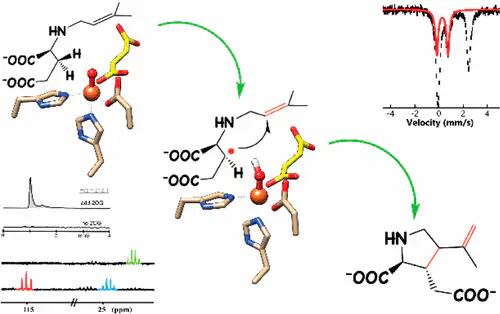
Metalloenzyme-catalyzed cyclization involving C–H bond activation is a powerful strategy to construct molecular complexity found in natural product biosynthesis. In the isodomoic acid and kainic acid biosynthetic pathways, mononuclear non-heme iron enzymes catalyze cyclization along with desaturation reactions that install the pyrrolidine and the olefin. Using complementary approaches, a plausible reaction pathway of kainic acid formation is established. Following H atom abstraction by an Fe(IV)-oxo species, the resulting radical interacts with the N-prenyl group to promote pyrrolidine installation. The reaction then undergoes a carbocation-triggered desaturation to construct kainic acid.
Type
Publication
ACS Catalysis
Supplementary Information is here, including data.