Mechanism of Methyldehydrofosmidomycin Maturation: Use Olefination to Enable Chain Elongation
Abstract
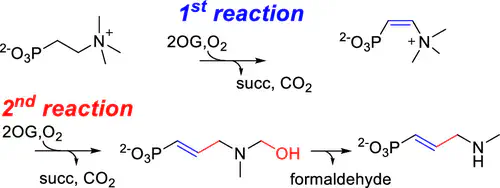
Utilization of mononuclear iron- and 2-oxoglutarate-dependent (Fe/2OG) enzymes to enable C–H bond functionalization is a widely used strategy to diversify the structural complexity of natural products. Besides those well-studied reactions including hydroxylation, epoxidation, and halogenation, in the biosynthetic pathway of dehydrofosmidomycin, an Fe/2OG enzyme is reported to catalyze desaturation, alkyl chain elongation, along with demethylation in which trimethyl-2-aminoethylphosphonate is converted into methyldehydrofosmidomycin. How this transformation takes place is largely unknown. Herein, we characterized the reactive species, revealed the structure of the reaction intermediate, and used mechanistic probes to investigate the reaction pathway and mechanism. These results led to the elucidation of a two-step process in which the first reaction employs a long-lived Fe(IV)-oxo species to trigger C═C bond installation. During the second reaction, the olefin installed in situ enables C–C bond formation that is accompanied with a C–N bond cleavage and hydroxylation to furnish the alkyl chain elongation and demethylation. This work expands the reaction repertoire of Fe/2OG enzymes by introducing a new pathway to the known C–C bond formation mechanisms utilized by metalloenzymes.
Supplementary Information is here, including data.