BesC Initiates C–C Cleavage through a Substrate-Triggered and Reactive Diferric-Peroxo Intermediate
Abstract
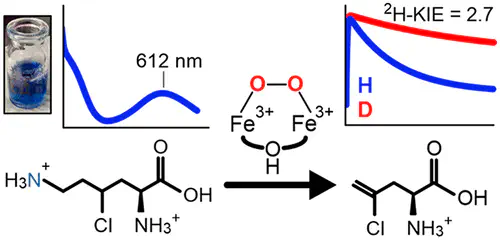
BesC catalyzes the iron- and O2-dependent cleavage of 4-chloro-l-lysine to form 4-chloro-l-allylglycine, formaldehyde, and ammonia. This process is a critical step for a biosynthetic pathway that generates a terminal alkyne amino acid which can be leveraged as a useful bio-orthogonal handle for protein labeling. As a member of an emerging family of diiron enzymes that are typified by their heme oxygenase-like fold and a very similar set of coordinating ligands, recently termed HDOs, BesC performs an unusual type of carbon–carbon cleavage reaction that is a significant departure from reactions catalyzed by canonical dinuclear-iron enzymes. Here, we show that BesC activates O2 in a substrate-gated manner to generate a diferric-peroxo intermediate. Examination of the reactivity of the peroxo intermediate with a series of lysine derivatives demonstrates that BesC initiates this unique reaction trajectory via cleavage of the C4–H bond; this process represents the rate-limiting step in a single turnover reaction. The observed reactivity of BesC represents the first example of a dinuclear-iron enzyme that utilizes a diferric-peroxo intermediate to capably cleave a C–H bond as part of its native function, thus circumventing the formation of a high-valent intermediate more commonly associated with substrate monooxygenations.
Supplementary Information is here, including data.